Multiple Choice
Identify the
choice that best completes the statement or answers the question.
|
|
|
1.
|
Which of the following are considered physical properties of a substance?
a. | color and odor | c. | malleability and hardness | b. | melting and boiling
points | d. | all of the
above |
|
|
|
2.
|
Which of the following is a physical change?
a. | corrosion | c. | evaporation | b. | explosion | d. | rotting of food |
|
|
|
3.
|
Which of the following is true about compounds?
a. | They can be physically separated into their component elements. | b. | They have
compositions that vary. | c. | They are substances. | d. | They have properties
similar to those of their component elements. |
|
|
|
4.
|
A substance that can be separated into two or more substances only by a chemical
change is a(n) ____.
a. | solution | c. | mixture | b. | element | d. | compound |
|
|
|
5.
|
What is one difference between a mixture and a compound?
a. | A compound consists of more than one phase. | b. | A compound can only
be separated into its components by chemical means. | c. | A mixture can only be separated into its
components by chemical means. | d. | A mixture must be uniform in
composition. |
|
|
|
6.
|
Which of the following is a chemical property?
a. | color | c. | freezing point | b. | hardness | d. | ability to react with
oxygen |
|
|
|
7.
|
All of the following changes to a metal are physical changes EXCEPT ____.
a. | bending | c. | rusting | b. | melting | d. | polishing |
|
|
|
8.
|
A chemical change occurs when a piece of wood ____.
a. | is split | c. | decays | b. | is painted | d. | is cut |
|
|
|
9.
|
Which of the following indicates that a chemical change has happened during
cooking?
a. | The food darkens. | b. | Bubbles form in boiling
water. | c. | Butter melts. | d. | Energy is transferred from the stove to a
pan. |
|
|
|
10.
|
When an iron nail is ground into powder, its mass ____.
a. | stays the same | c. | increases | b. | decreases | d. | cannot be
determined |
|
|
|
11.
|
The closeness of a measurement to its true value is a measure of its
____.
a. | precision | c. | reproducibility | b. | accuracy | d. | usefulness |
|
|
|
12.
|
Which of the following measurements contains two significant figures?
a. | 0.004 00 L | c. | 0.000 44 L | b. | 0.004 04 L | d. | 0.004 40 L |
|
|
|
13.
|
How many significant figures are in the measurement 811.40 grams?
|
|
|
14.
|
Express the sum of 7.68 m and 5.0 m using the correct number of significant
digits.
a. | 12.68 m | c. | 13 m | b. | 12.7 m | d. | 10 m |
|
|
|
15.
|
Which of the following is true about subatomic particles?
a. | Electrons are negatively charged and are the heaviest subatomic
particle. | b. | Protons are positively charged and the lightest subatomic
particle. | c. | Neutrons have no charge and are the lightest subatomic particle. | d. | The mass of a
neutron nearly equals the mass of a proton. |
|
|
|
16.
|
The nucleus of an atom is ____.
a. | the central core and is composed of protons and neutrons | b. | positively charged
and has more protons than neutrons | c. | negatively charged and has a high
density | d. | negatively charged and has a low density |
|
|
|
17.
|
The atomic number of an element is the total number of which particles in the
nucleus?
a. | neutrons | c. | electrons | b. | protons | d. | protons and
electrons |
|
|
|
18.
|
An element has an atomic number of 76. The number of protons and electrons in a
neutral atom of the element are ____.
a. | 152 protons and 76 electrons | c. | 38 protons and 38
electrons | b. | 76 protons and 0 electrons | d. | 76 protons and 76 electrons |
|
|
|
19.
|
All atoms of the same element have the same ____.
a. | number of neutrons | c. | mass numbers | b. | number of protons | d. | mass |
|
|
|
20.
|
How does the energy of an electron change when the electron moves closer to the
nucleus?
a. | It decreases. | c. | It stays the same. | b. | It increases. | d. | It doubles. |
|
|
|
21.
|
When an electron moves from a lower to a higher energy level, the electron
____.
a. | always doubles its energy | b. | absorbs a continuously variable amount of
energy | c. | absorbs a quantum of energy | d. | moves closer to the
nucleus |
|
|
|
22.
|
The atomic emission spectra of a sodium atom on Earth and of a sodium atom in
the sun would be ____.
a. | the same | b. | different from each other | c. | the same as those of
several other elements | d. | the same as each other only in the ultraviolet
range |
|
|
|
23.
|
Which of the following categories includes the majority of the elements?
a. | metalloids | c. | metals | b. | liquids | d. | nonmetals |
|
|
|
24.
|
Of the elements Pt, V, Li, and Kr, which is a nonmetal?
|
|
|
25.
|
To what category of elements does an element belong if it is a poor conductor of
electricity?
a. | transition elements | c. | nonmetals | b. | metalloids | d. | metals |
|
|
|
26.
|
What element has the electron configuration 1 s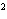 2 s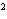 2 p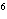 3 s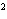 3 p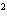 ? a. | nitrogen | c. | silicon | b. | selenium | d. | silver |
|
|
|
27.
|
What is the charge on the strontium ion?
a. | 2– | c. | 1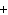 | b. | 1– | d. | 2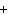 |
|
|
|
28.
|
Which of the following is true about an ionic compound?
a. | It is a salt. | c. | It is composed of anions and cations. | b. | It is held together
by ionic bonds. | d. | all of the
above |
|
|
|
29.
|
What is the formula unit of sodium nitride?
|
|
|
30.
|
Which of the following pairs of elements is most likely to form an ionic
compound?
a. | magnesium and fluorine | b. | nitrogen and sulfur | c. | oxygen and
chlorine | d. | sodium and aluminum |
|
|
|
31.
|
Ionic compounds are normally in which physical state at room temperature?
a. | solid | c. | gas | b. | liquid | d. | plasma |
|
|
|
32.
|
Which of the following is true about the melting temperature of potassium
chloride?
a. | The melting temperature is relatively high. | b. | The melting
temperature is variable and unpredictable. | c. | The melting temperature is relatively
low. | d. | Potassium chloride does not melt. |
|
|
|
33.
|
What characteristic of metals makes them good electrical conductors?
a. | They have mobile valence electrons. | b. | They have mobile protons. | c. | They have mobile
cations. | d. | Their crystal structures can be rearranged easily. |
|
|
|
34.
|
What is the correct name for the N 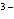
ion? a. | nitrate ion | c. | nitride ion | b. | nitrogen ion | d. | nitrite ion |
|
|
|
35.
|
Which of the following compounds contains the Mn 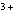 ion?
|
|
|
36.
|
Which of the following formulas represents an ionic compound?
|
|
|
37.
|
What is the correct formula for potassium sulfite?
|
|
|
38.
|
What is the ending for the names of all binary compounds, both ionic and
molecular?
|
|
|
39.
|
Select the correct formula for sulfur hexafluoride.
|
|
|
40.
|
What is the correct formula for barium chlorate?
a. | Ba(ClO)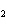 | c. | Ba(ClO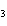 ) )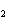 | b. | Ba(ClO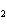 ) )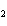 | d. | BaCl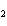 |
|
|
|
41.
|
How many moles of silver atoms are in 1.8 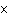 10 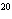 atoms of silver?
|
|
|
42.
|
Chemical equations ____.
a. | describe chemical reactions | b. | show how to write chemical
formulas | c. | give directions for naming chemical compounds | d. | describe only
biological changes |
|
|
|
43.
|
What are the coefficients that will balance the skeleton equation below?
N 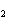 + H 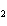  NH 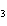 a. | 1, 1, 2 | c. | 3, 1, 2 | b. | 1, 3, 3 | d. | 1, 3, 2 |
|
|
|
44.
|
Which of the following statements is NOT true about what happens in all chemical
reactions?
a. | The ways in which atoms are joined together are changed. | b. | New atoms are formed
as products. | c. | The starting substances are called reactants. | d. | The bonds of the
reactants are broken and new bonds of the products are formed. |
|
|
|
45.
|
In every balanced chemical equation, each side of the equation has the same
number of ____.
a. | atoms of each element | c. | moles | b. | molecules | d. | coefficients |
|
|
|
46.
|
What are the missing coefficients for the skeleton equation
below? Cr( s) 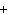 Fe(NO 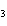 ) 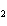 ( aq) 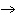 Fe( s) 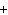 Cr(NO 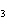 ) 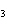 ( aq) a. | 4, 6, 6, 2 | c. | 2, 3, 3, 2 | b. | 2, 3, 2, 3 | d. | 1, 3, 3, 1 |
|
|
|
47.
|
In a combustion reaction, one of the reactants is ____.
a. | hydrogen | c. | oxygen | b. | nitrogen | d. | a metal |
|
|
|
48.
|
In a chemical reaction, the mass of the products ____.
a. | is less than the mass of the reactants | b. | is greater than the mass of the
reactants | c. | is equal to the mass of the reactants | d. | has no relationship to the mass of the
reactants |
|
|
|
49.
|
Aluminum reacts with sulfuric acid to produce aluminum sulfate and hydrogen gas.
How many grams of aluminum sulfate would be formed if 250 g H 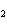 SO 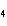 completely reacted with
aluminum? 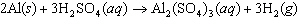 a. | 0.85 g | c. | 450 g | b. | 290 g | d. | 870 g |
|
|
|
50.
|
How many grams of beryllium are needed to produce 36.0 g of hydrogen? (Assume an
excess of water.) Be( s) + 2H 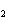 O( l) 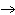 Be(OH) 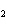
( aq) + H 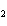 ( g) a. | 4.00 g | c. | 162 g | b. | 36.0 g | d. | 324 g |
|
|
|
51.
|
The rate of a chemical reaction normally ____.
a. | decreases as temperature increases | b. | is slowed down by a
catalyst | c. | increases as reactant concentration increases | d. | decreases as
reactant concentration increases |
|