Matching
|
|
|
Match each item with the correct statement below. a. | mixture | d. | reactant | b. | product | e. | heterogeneous mixture | c. | phase | f. | vapor |
|
|
|
1.
|
gaseous state of substance that is a liquid or solid at room
temperature
|
|
|
2.
|
a physical blend of two or more components
|
|
|
3.
|
part of a sample having uniform composition and properties
|
|
|
4.
|
not uniform in composition
|
|
|
5.
|
a substance formed in a chemical reaction
|
|
|
6.
|
starting substance in a chemical reaction
|
|
|
Match each item with the correct statement below. a. | distillation | d. | compound | b. | mass | e. | element | c. | chemical
reaction | f. | homogeneous
|
|
|
|
7.
|
amount of matter an object contains
|
|
|
8.
|
describes mixture with a uniform composition
|
|
|
9.
|
a process in which a liquid is boiled to produce a vapor that is condensed
again into a liquid
|
|
|
10.
|
substance that cannot be changed into simpler substances by chemical
means
|
|
|
11.
|
composed of two or more substances chemically combined in a fixed
proportion
|
|
|
12.
|
process in which substances are changed into different substances
|
|
|
Match each item with the correct statement below. a. | absolute zero | e. | mass | b. | Kelvin temperature scale | f. | significant figure | c. | Celsius temperature
scale | g. | precision | d. | weight | h. | accuracy |
|
|
|
13.
|
closeness to true value
|
|
|
14.
|
narrowness of range of measurements
|
|
|
15.
|
known or estimated in a measurement
|
|
|
16.
|
the quantity of matter an object contains
|
|
|
17.
|
the lowest point on the Kelvin scale
|
|
|
18.
|
the SI scale for temperature
|
|
|
19.
|
the force of gravity on an object
|
|
|
20.
|
the non-SI scale for temperature
|
Multiple Choice
Identify the
choice that best completes the statement or answers the question.
|
|
|
21.
|
Which of the following is NOT an example of matter?
a. | air | c. | smoke | b. | heat | d. | water vapor |
|
|
|
22.
|
An example of an extensive property of matter is ____.
a. | temperature | c. | mass | b. | pressure | d. | hardness |
|
|
|
23.
|
All of the following are physical properties of matter EXCEPT ____.
a. | mass | c. | melting point | b. | color | d. | ability to rust |
|
|
|
24.
|
Which of the following is NOT a physical property of water?
a. | It has a boiling point of 100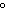 C. C. | b. | It is a colorless
liquid. | c. | It is composed of hydrogen and oxygen. | d. | Sugar dissolves in
it. |
|
|
|
25.
|
Which of the following is a physical change?
a. | corrosion | c. | evaporation | b. | explosion | d. | rotting of food |
|
|
|
26.
|
Which of the following is a heterogeneous mixture?
a. | air | c. | steel | b. | salt water | d. | soil |
|
|
|
27.
|
Which of the following is true about homogeneous mixtures?
a. | They are known as solutions. | b. | They consist of two or more
phases. | c. | They have compositions that never vary. | d. | They are always
liquids. |
|
|
|
28.
|
Which of the following is a homogeneous mixture?
a. | salt water | c. | sand and water | b. | beef stew | d. | soil |
|
|
|
29.
|
Separating a solid from a liquid by evaporating the liquid is called
____.
a. | filtration | c. | solution | b. | condensation | d. | distillation |
|
|
|
30.
|
Which of the following items is NOT a compound?
a. | baking soda | c. | sucrose | b. | salad dressing | d. | table salt |
|
|
|
31.
|
A substance that can be separated into two or more substances only by a chemical
change is a(n) ____.
a. | solution | c. | mixture | b. | element | d. | compound |
|
|
|
32.
|
What is one difference between a mixture and a compound?
a. | A compound consists of more than one phase. | b. | A compound can only
be separated into its components by chemical means. | c. | A mixture can only be separated into its
components by chemical means. | d. | A mixture must be uniform in
composition. |
|
|
|
33.
|
Which of the following is a chemical property?
a. | color | c. | freezing point | b. | hardness | d. | ability to react with
oxygen |
|
|
|
34.
|
What must occur for a change to be a chemical reaction?
a. | There must be a change in chemical properties. | b. | There must be a
change in physical properties. | c. | The change must involve a change in
mass. | d. | The change must involve a change in volume. |
|
|
|
35.
|
Which of the following is NOT a physical change?
a. | grating cheese | c. | fermenting of cheese | b. | melting cheese | d. | mixing two cheeses in a
bowl |
|
|
|
36.
|
All of the following changes to a metal are physical changes EXCEPT ____.
a. | bending | c. | rusting | b. | melting | d. | polishing |
|
|
|
37.
|
A chemical change occurs when a piece of wood ____.
a. | is split | c. | decays | b. | is painted | d. | is cut |
|
|
|
38.
|
Which of the following indicates that a chemical change has happened during
cooking?
a. | The food darkens. | b. | Bubbles form in boiling
water. | c. | Butter melts. | d. | Energy is transferred from the stove to a
pan. |
|
|
|
39.
|
When an iron nail is ground into powder, its mass ____.
a. | stays the same | c. | increases | b. | decreases | d. | cannot be
determined |
|
|
|
40.
|
The diameter of a carbon atom is 0.000 000 000 154 m. What is this number
expressed in scientific notation?
|
|
|
41.
|
The expression of 5008 km in scientific notation is ____.
|
|
|
42.
|
The closeness of a measurement to its true value is a measure of its
____.
a. | precision | c. | reproducibility | b. | accuracy | d. | usefulness |
|
|
|
43.
|
Which of the following measurements contains two significant figures?
a. | 0.004 00 L | c. | 0.000 44 L | b. | 0.004 04 L | d. | 0.004 40 L |
|
|
|
44.
|
When a test instrument is calibrated, does its accuracy, precision, or
reliability improve?
a. | precision | c. | reliability | b. | accuracy | d. | all of the
above |
|
|
|
45.
|
Which group of measurements is the most precise? (Each group of measurements is
for a different object.)
a. | 2 g, 3 g, 4 g | c. | 2 g, 2.5 g, 3 g | b. | 2.0 g, 3.0 g, 4.0 g | d. | 1 g, 3 g, 5 g |
|
|
|
46.
|
Which of the following measurements is expressed to three significant
figures?
a. | 0.007 m | c. | 7.30 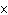 10 10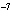 km
km | b. | 7077 mg | d. | 0.070 mm |
|
|
|
47.
|
In the measurement 0.503 L, which digit is the estimated digit?
a. | 5 | b. | the 0 immediately to the left of the
3 | c. | 3 | d. | the 0 to the left of the decimal
point |
|
|
|
48.
|
Express the product of 2.2 mm and 5.00 mm using the correct number of
significant digits.
a. | 10 mm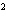 | c. | 11.0 mm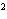 | b. | 11 mm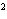 | d. | 11.00
mm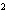 |
|
|
|
49.
|
What is the measurement 111.009 mm rounded off to four significant
digits?
a. | 111 mm | c. | 111.01 mm | b. | 111.0 mm | d. | 110 mm |
|
|
|
50.
|
When multiplying and dividing measured quantities, the number of significant
figures in the result should be equal to the number of significant figures in ____.
a. | all of the measurements | b. | the least and most precise
measurements | c. | the most precise measurement | d. | the least precise
measurement |
|