Multiple Choice
Identify the
choice that best completes the statement or answers the question.
|
|
|
1.
|
What is conserved in the reaction shown below? H 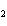 ( g) + Cl 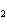 ( g) ® 2HCl( g) a. | mass only | c. | mass, moles, and molecules only | b. | mass and moles
only | d. | mass, moles,
molecules, and volume |
|
|
|
2.
|
How many moles of aluminum are needed to react completely with 1.2 mol of
FeO? 2Al( s) + 3FeO( s) ® 3Fe( s) + Al 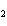 O 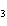 ( s) a. | 1.2 mol | c. | 1.6 mol | b. | 0.8 mol | d. | 2.4 mol |
|
|
|
3.
|
The calculation of quantities in chemical equations is called ____.
a. | stoichiometry | c. | percent composition | b. | dimensional analysis | d. | percent yield |
|
|
|
4.
|
How many atoms are in 0.075 mol of titanium?
|
|
|
5.
|
What is the mass in grams of 5.90 mol C 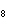 H 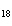 ? a. | 0.0512 g | c. | 389 g | b. | 19.4 g | d. | 673 g |
|
|
|
6.
|
If 1 egg and 1/3 cup of oil are needed for each bag of brownie mix, how many
bags of brownie mix do you need if you want to use up all 3 eggs and 1 cup of oil?
|
|
|
7.
|
How many moles of tungsten atoms are in 4.8 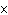 10 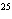 atoms of tungsten?
|
|
|
8.
|
Aluminum reacts with sulfuric acid to produce aluminum sulfate and hydrogen gas.
How many grams of aluminum sulfate would be formed if 250 g H 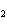 SO 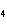 completely reacted with
aluminum? 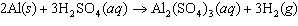 a. | 0.85 g | c. | 450 g | b. | 290 g | d. | 870 g |
|
|
|
9.
|
In the reaction 2CO( g) + O  ( g) ® 2CO 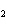 ( g), what is the ratio of moles
of oxygen used to moles of CO 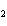 produced?
|
|
|
10.
|
The equation below shows the decomposition of lead nitrate. How many grams of
oxygen are produced when 11.5 g NO 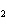 is formed? 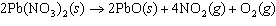 a. | 1.00 g | c. | 2.88 g | b. | 2.00 g | d. | 32.0 g |
|
|
|
11.
|
The first step in most stoichiometry problems is to ____.
a. | add the coefficients of the reagents | c. | convert given quantities to
volumes | b. | convert given quantities to moles | d. | convert given quantities to
masses |
|
|
|
12.
|
What is the number of moles of beryllium atoms in 36 g of Be?
a. | 0.25 mol | c. | 45.0 mol | b. | 4.0 mol | d. | 320 mol |
|
|
|
13.
|
Which of the following is true about the total number of reactants and the total
number of products in the reaction shown below? C 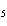 H 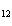 ( l) + 8O 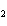 ( g) ® 5CO 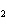 ( g) + 6H 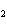 O( g) a. | 9 moles of reactants chemically change into 11 moles of product. | b. | 9 grams of reactants
chemically change into 11 grams of product. | c. | 9 liters of reactants chemically change into 11
liters of product. | d. | 9 atoms of reactants chemically change into 11
atoms of product. |
|
|
|
14.
|
What is the molar mass of AuCl3?
a. | 96 g | c. | 232.5 g | b. | 130 g | d. | 303.6 g |
|